Pressemitteilung der Autonomen Universität Barcelona (UAB) vom 05.09.2022
Researchers from the Bacterial Pathogenesis and Antimicrobials group of the Institut de Biotecnologia i de Biomedicina (IBB) and the Department of Genetics and Microbiology at UAB, have evaluated several compounds that have been shown to inhibit biofilm formation in important opportunistic human pathogens. The targets of these molecules include multidrug-resistant Gram-negative and Gram-positive pathogenic bacteria as well as the fungal pathogen Candida albicans. The study was carried out in a collaboration which involved three more European laboratories.
Biofilm producing bacteria are a serious threat to public health due to, among other things, their high resistance to antimicrobials. In addition, biofilm formation represents an important virulence factor because it allows pathogens to evade host defences, provides adhesion and mechanical integrity to microbial populations, and creates microenvironments for nutrient and metabolite exchange and cell-to-cell communication. Quorum-sensing (QS)-mediated cell signalling is known to regulate biofilm formation during host colonization and chronic infections. Accordingly, all the elements that take part in the regulation of QS systems could potentially be attractive targets to combat antibiotic-resistant biofilm-forming bacteria.
Biofilms are polymicrobial in nature and cell-to-cell communication systems participate in increasing the resistance of the most susceptible co-occupants. Intra- and inter-kingdom (e.g., bacteria/fungi) biofilms, such as Pseudomonas aeruginosa/Staphylococcus aureus or P. aeruginosa/C. albicans, have been identified in infections of the lungs, skin and medical devices leading to a medical concern. For example, patients with cystic fibrosis go through a more difficult time to recover from mixed-species lung infections than those who are infected by single species. One of the recent lines of research of our group at the IBB is related to the study of mixed bacterial biofilms.
Some natural products with antimicrobial and antibiofilm activity have been shown to interfere with bacterial QS systems. For example, furanones isolated from Australian seaweed interfere with the activity of QS systems based on AHL- and AI-2-type signal molecules. The autoinducers AHL and AI-2 are suspected to be universal or broad-spectrum molecules in cell-to-cell communication because they are produced and sensed by many different microorganisms. Many halogenated furanones, and related synthetic analogues, have been discovered to inhibit biofilm formation in a variety of pathogens. Our most recent work is framed in this context.
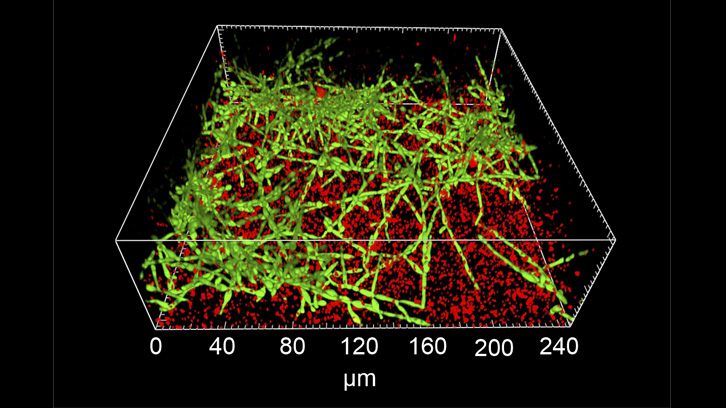
In this work, which was possible thanks to an international collaboration with Dr. Timothy P O'Sullivan (School of Pharmacy, University College Cork, Ireland), Dr. Uwe Mamat (Research Center Borstel, Germany) and Dr. Sascha Brunke (Hans Knoell Institute, Jena, Germany), a diverse library of dihalofuranones synthesised in Dr. O'Sullivan's laboratory was evaluated. The novel furanones were tested as biofilm inhibitors in several opportunistic human pathogens including S. aureus, P. aeruginosa, Salmonella enterica, Escherichia coli, Stenotrophomonas maltophilia, and C. albicans. Selected furanones were subjected to further analysis using confocal laser-scanning microscopy in Dr. Mamat's laboratory for their effect on single-species and mixed biofilms of C. albicans and P. aeruginosa. Finally, the most promising candidate compound showing no toxicity in human cells, led to an increase in survival rates in the Galleria mellonella (wax moth) acute infection model. This work has been published in European Journal of Medicinal Chemistry.
Ansprechperson:
Institut de Biotecnologia i de Biomedicina (IBB)
Department of Genetics and Microbiology
Universitat Autònoma de Barcelona
Originalpublikation:
Gómez AC, Lyons T, Mamat U, Yero D, Bravo M, Daura X, Elshafee O, Brunke S, Gahan CGM, O'Driscoll M, Gibert I, O'Sullivan TP. 2022. Synthesis and evaluation of novel furanones as biofilm inhibitors in opportunistic human pathogens. Eur J Med Chem. 242:114678. doi: 10.1016/j.ejmech.2022.114678. https://pubmed.ncbi.nlm.nih.gov/36037789/